Be More Than a Physician. Be an Investigator.
Advance Patient Care, Contribute to Innovation, and Grow Professionally.
Partnerships to Support Clinical Research Physicians & Sites
Whether you’re new to clinical research or looking to expand your capabilities, Revival Research Institute partners with physicians ready to take the next step. Our expert team manages every aspect of site activation—from startup and regulatory compliance to patient recruitment, training, and data operations—so you can focus on what matters most: exceptional patient care and clinical excellence.
With a growing network of over 30 investigators, we’re committed to improving lives by bringing cutting-edge therapies directly to communities. Join us in making clinical research more accessible, without the administrative burden.
0+
Studies Completed

Contribute to Advancement in Medicine and Reap the Benefits
One of the many advantages of collaborating with Revival Research Institute is gaining firsthand experience with cutting-edge technologies and investigational treatments that you may be able to offer your patients. Through clinical research, your patients can gain access to advanced therapies that are otherwise unavailable in standard care.
Physicians and principal investigators engaged in research are often regarded as leaders in their field, trusted by patients and recognized by peers. Partnering with Revival connects you to hundreds of pharmaceutical and biotechnology sponsors, a broad pipeline of clinical studies, and a dedicated support team that handles startup, regulatory, and operational complexity.
Join a research movement that elevates your clinical career while improving lives; starting with your own patients.

Endless Possibilities
Why Join Revival’s Site Network?
At Revival Research Institute, we support our clinical investigators every step of the way. Whether you’re new to research or a seasoned PI, we offer tailored assistance to help you thrive in every trial you conduct.
Be a Part of the Next Breakthrough
As a clinical research physician, you have the unique opportunity to shape the future of medicine through exclusive trials tailored to your research interests. Whether you are driven by helping your patients or advancing medicine, you could help create the next breakthrough.

Financial Compensation
Working as Revival's clinical research physician allows you to reduce administrative burden, lower costs, and improve the quality of your clinical trials. We want you to focus on what you do best, helping patients by providing them access to research as a care option.
Gain Access to the Latest in Medical Research
Partnering with a clinical research company like Revival Research Institute provides opportunities to work with hundreds of pharmaceutical and biotechnology organizations, as well as exposure to a variety of research projects.
Research as a Care Option
Joining Revival's Principal Investigator network offers you the opportunity to provide access to research as a care option for patients. With exclusive clinical trials cherry-picked to meet your patient's interests, the possibilities are endless.

Working with Revival
Pillars of Our Institution
Our Growing Investigator’s Network
Revival Research Institute collaborates with primary care and specialty physicians to bring advanced clinical trials to diverse patient populations. With a growing national network of experienced Principal and Sub-Investigators, we aim to improve access to novel therapies and advance patient care through research-driven innovation.
By partnering with Revival, you’ll gain access to a full suite of clinical research services designed to streamline operations and accelerate timelines. Our experienced research staff ensures efficient study start-ups, regulatory compliance, and consistent quality of patient data. Outsourcing critical functions like recruitment and quality assurance not only saves time and cost but also enhances your financial returns. Together, we can improve health outcomes and shape the future of medicine.
Sponsors We Work With
Contributing to the Changing Clinical Research Landscape
Revival Research Institute’s success and innovative approach have made it possible for us to keep on working with leading sponsors and CROs to make novel therapies accessible through clinical trials.






Reducing Risks & Executing Efficient Clinical Trials
Our Track Record
Striving towards the goal of operational excellence, we’ve streamlined our processes and executed stringent timelines to ensure the quality of your clinical trials and the efficiency of the trial lifecycle, from start to finish. Here are our metrics.
0+
Years of Expertise
0+
Investigators
0+
Dedicated Employees
0+
Patient Engaged
Successful Outcomes
Our Operational Excellence is Measurable
At Revival Research Institute, every study begins with a purpose, understanding the root cause of a condition and exploring new ways to treat it. Our case studies highlight the journey from trial conception to real-world impact, offering insight into how clinical research transforms patient care and drives medical innovation.
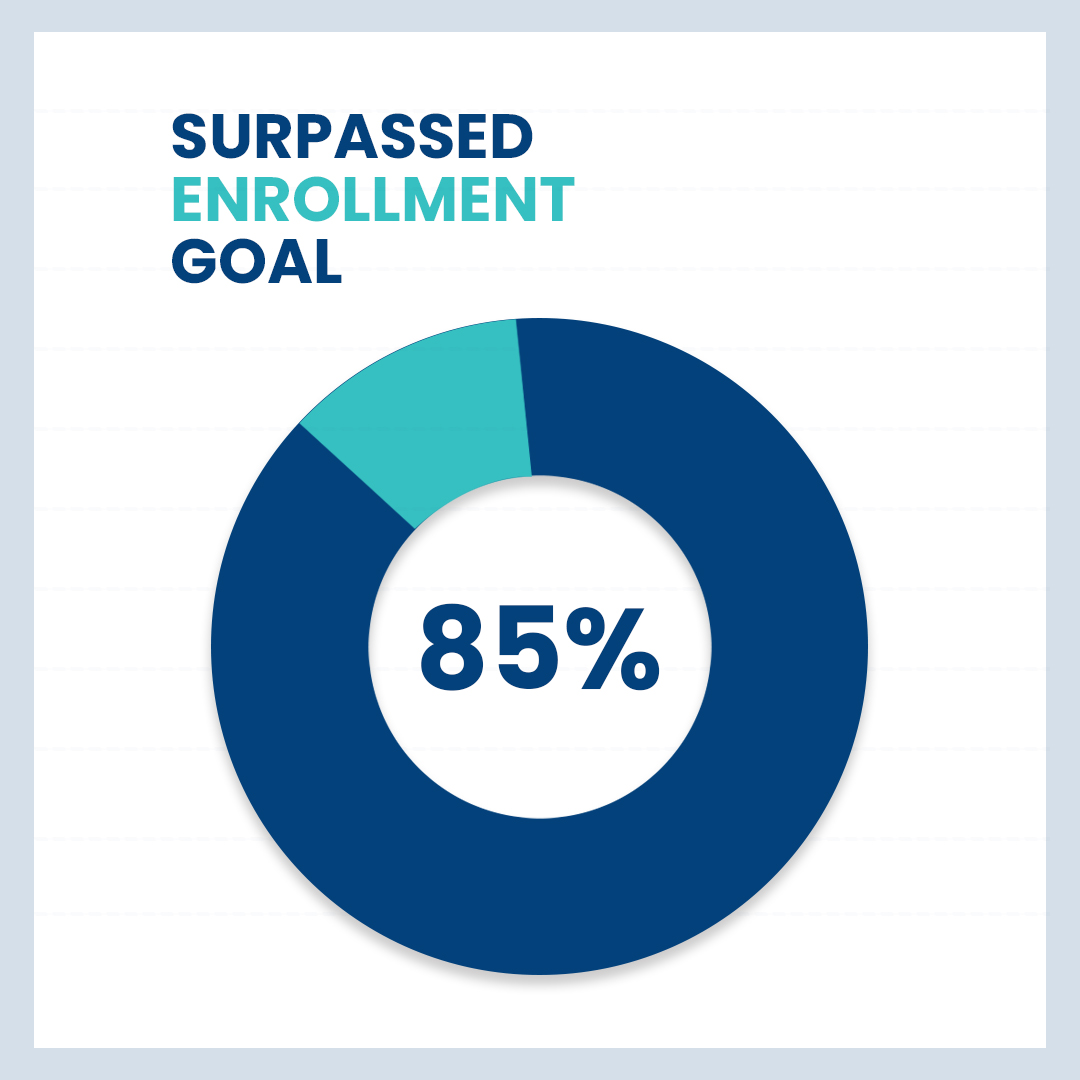
Dermatology
PRURIGO NODULARIS (PN)
With tough study enrollment criteria, we were able to exceed enrollment goals by up to 85% for this study. Our Dermatology site in Troy, Michigan, was recognized as one of the highest recruiting for PN Clinical Trials throughout the world, by the Sponsor.
Internal Medicine
mRNA FLU VACCINE
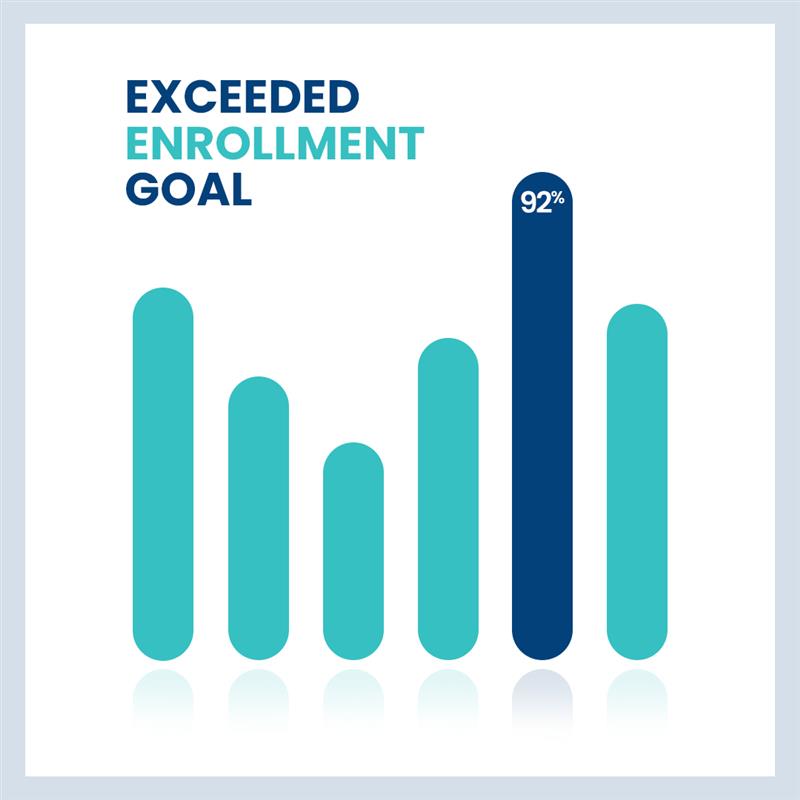
In 2022, we were tasked with recruiting a set number of participants within a 6-months time frame. We were able to exceed enrollment goals by up to 92%. The targeted population involved 2 age groups, younger & older, to test the efficacy of the vaccine in both age groups.
Internal Medicine
PREVENTIVE ALZHEIMER’S
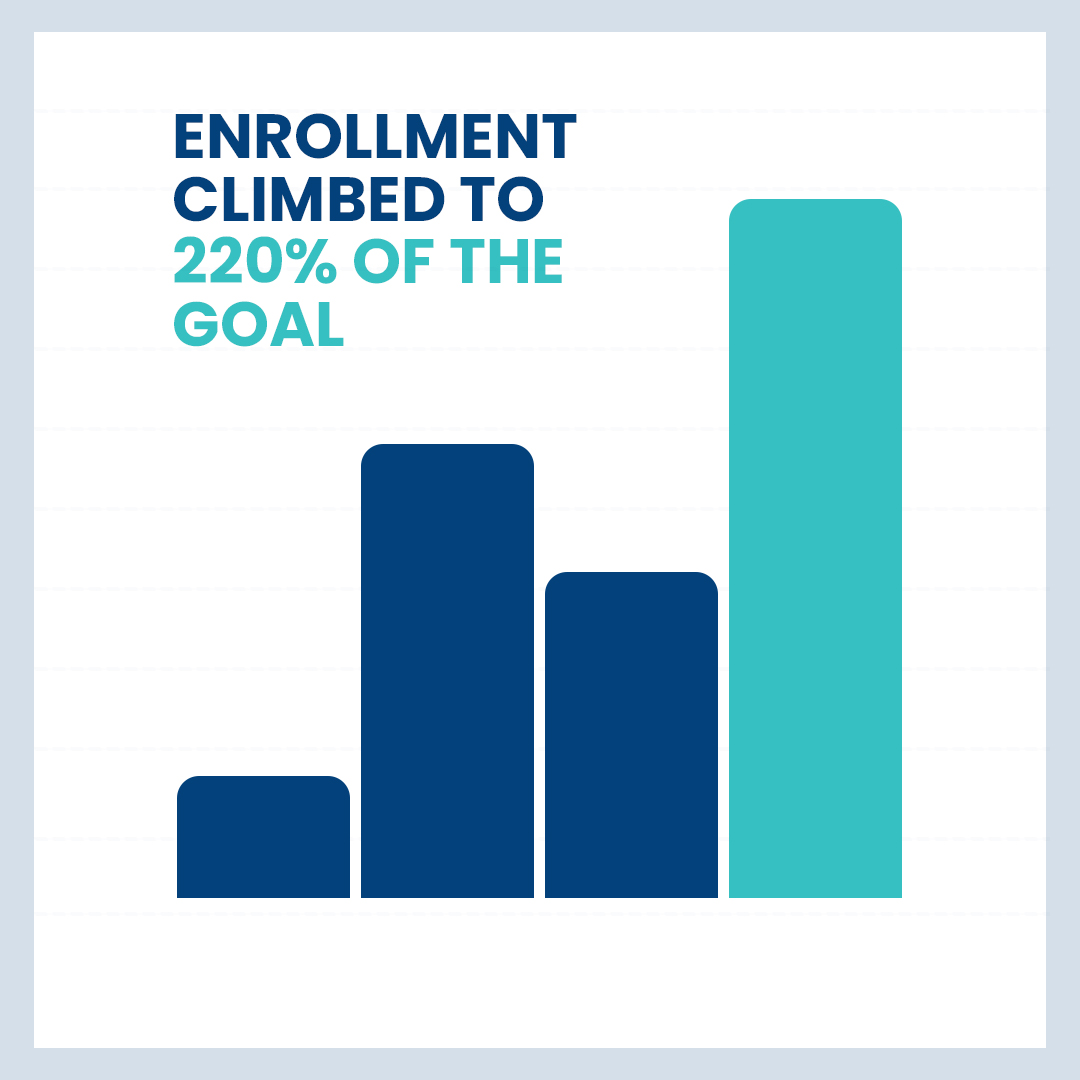
Our Preventive Alzheimer’s study was a one of its kind preventive studies in which we recruited participants between the age group of 65-80 years who had a risk of Alzheimer’s disease but not the disease itself. We successfully exceeded our enrollment goals by more than 220%.
For Physicians
Let’s Advance Clinical Research,,
Together
Partner with Revival Research Institute and experience fast study startups, seamless execution, and data you can trust. Connect with us today to explore how we can support your next trial.


